Newly added export medical device commodity inspection, enterprises should know these
——Pre-export preparation
01
Business license .
02
Obtain the domestic medical device production license and the medical device product registration/filing certificate .

If the importing country (region) requires it, the manufacturer must also obtain the record of the medical device manufacturer from the drug regulatory authority and apply for a "Medical Device Product Export Sales Certificate . " Manufacturers also need to meet other management requirements of the drug regulatory department .
03
Obtain the right to import and export operations. The local commerce department handles the registration procedures for foreign trade operators and obtains the "Foreign Trade Operators Record Registration Form" .
If there is no import and export right, it can also be sold to an enterprise that has the right to export and import.
04
After the enterprise obtains the right to operate import and export, it shall go through the registration procedures at the customs and obtain the "Customs Registration Certificate of the Consignor and Consignor of Import and Export Goods of the People's Republic of China .
Obtain the technical ability to export
The factory must have technical capabilities that meet the requirements of foreign access regulations and standards.
The domestic and international quality and safety standards and access requirements for medical supplies can be inquired on the "General Administration of Customs Website-General Administration General Administration-Commodity Inspection Department-Policies and Regulations" column (updated from time to time).
address:
http://sjs.customs.gov.cn/sjs/zcfg56/index.html
Tip 1:
The types of medical device products and foreign regulations and standards are more complicated. Taking masks exported to the United States as an example, exports to the United States require FDA registration.
(1) US FDA registration:
https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfRL/rl.cfm
FDA 510(K) pre-marketing registration is a pre-marketing report submitted to the FDA. According to FDA requirements, a 510(K) application must be submitted at least 90 days in advance for a small number of Class I and most Class II medical devices before they are marketed in the United States. Devices used for busy sales are at least as safe and effective as legally sold devices 510 (k) Regulations (21 CFR 807.92(a)(3)). The applicant cannot be sold in the United States until the FDA declares that the device is "substantially equivalent" (SE) in a new format instruction. 510(k) application materials must include other information such as product code, label, summary of device technical characteristics, test results, etc. 510(k) does not have a fixed format or template, but all information must meet the requirements of 21 CFR Section 807.
(2) The FDA must designate a US citizen (company/society) as its agent when registering. This agent is responsible for the process services in the United States and is the medium for contacting the FDA and the applicant. FDA registration does not have a certificate. After a product is registered with the FDA, a registration number will be obtained. The FDA will give the applicant a reply letter (with the signature of the FDA chief executive), but there is no FDA certificate.
(3) At present, there are many domestic agent registration agencies, and export enterprises should find reputable registration agencies. For example, China Certification and Inspection Group, which is a professional third-party quality service organization, provides services such as domestic registration, EU CE, and US FDA/NIOSH registration for companies' epidemic prevention products (such as masks, protective clothing, infrared thermometer testing).
China Inspection and Quarantine's main anti-epidemic product service capabilities
(4) On April 14, the U.S. FDA issued a list of Chinese manufacturers of "authorized import" masks. 74 mask manufacturers in China have received emergency use authorization (EUA), most of which are local Chinese manufacturers (such as BYD). Precision Manufacturing Co., Ltd., Ogilvy Medical Products Co., Ltd., China Nano Technology Co., Ltd., Guangzhou Aiyin Co., Ltd., Kinner Medical Equipment Co., Ltd., etc.), including wholly foreign-owned enterprises (such as: 3M China). The specific list is as follows:
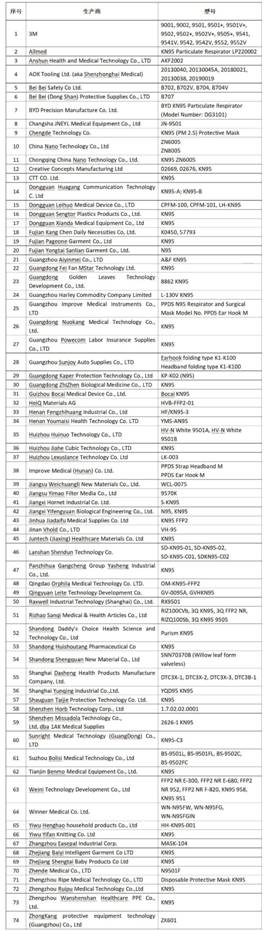
(Note: The list will be constantly updated, the authorized company link https://www.fda.gov/media/136663/download)
To obtain EUA authorization for masks produced in China, three principles need to be met: one is the manufacturer with one or more NIOSH (National Institute of Occupational Safety and Health) certified products, and others are manufactured in accordance with the applicable authorization standards of other countries/regions. Models of filter face mask respirators (FFR) can be verified by the FDA; the second is authorized by other regions outside of China, and the FDA can verify; third, there is a test report issued by an independent testing laboratory that can show that the product performance meets Applicable test standards can be verified by FDA.
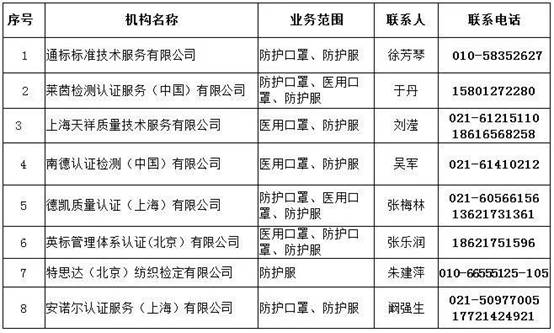
Tip 2:
List of certification bodies in China with EU-recognized CE certification capabilities for anti-epidemic supplies such as masks
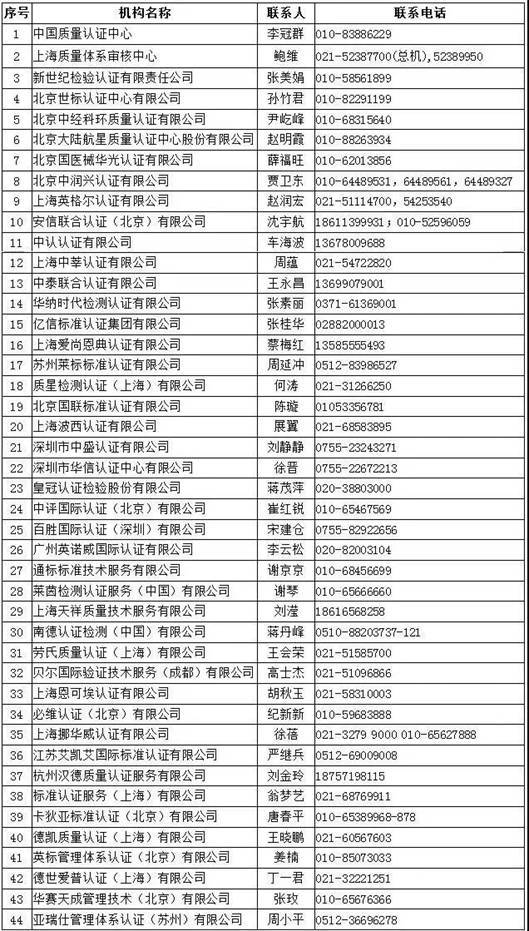
Tip 3:
List of institutions in China that can carry out medical device management system (ISO13485) certification
Understand my country's customs export policies
01
The "Announcement No. 5" jointly issued by the Ministry of Commerce, the General Administration of Customs, and the National Medical Products Administration states that the scope of the export of medical supplies covers the export of new coronavirus detection reagents, medical masks, medical protective clothing, ventilators, and infrared thermometers.

02
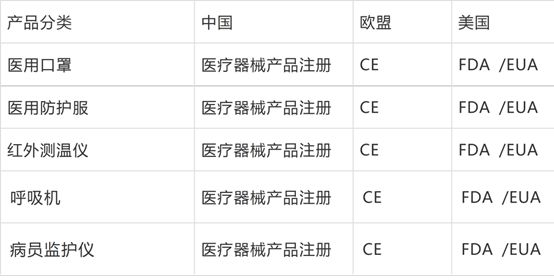
The 11 categories of medical materials listed in the "Announcement No. 53" of the General Administration of Customs are included in the legal inspection of commodities (see attachment).

Annex to Announcement No. 53 (2020) of the General Administration of Customs:
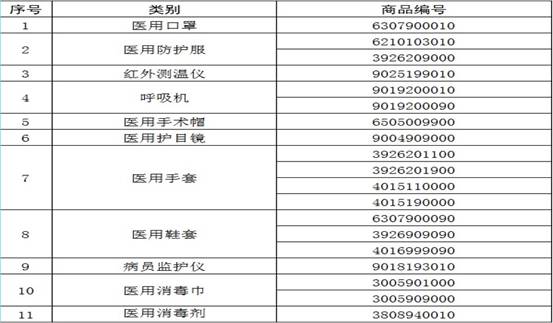
03
Pre-shipment inspection by intergovernmental agreement.
According to the bilateral agreements signed between the Chinese government and the governments of Sierra Leone, Ethiopia, Iran, Yemen and other countries, commodities exported to Sierra Leone, Ethiopia, Iran, Yemen and within the scope of pre-shipment inspection should apply to the customs for pre-shipment inspection. The scope of goods inspected before shipment is as follows:
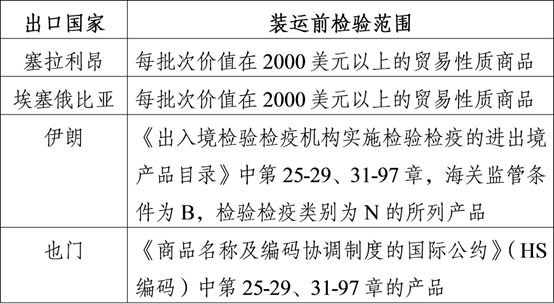
Commodities included in the scope of pre-shipment inspection, whether they are medical materials or non-medical materials, should apply for pre-shipment inspection.


